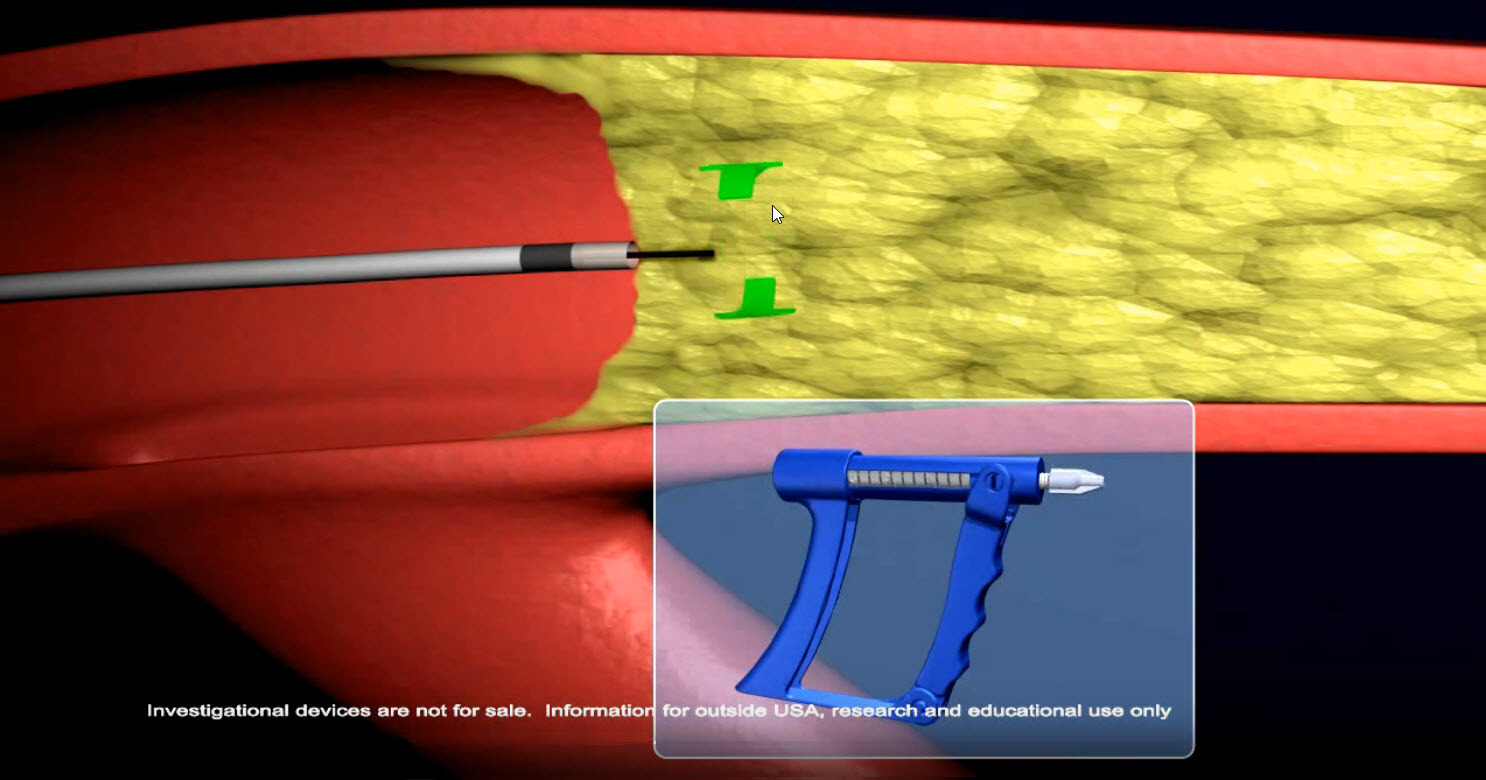
INDICATIONS FOR USE: The SPINR are used to maneuver guide wires in the coronary and peripheral vasculature. SPINRs are not intended for use in the neurovasculature. SPINR devices are “tools” not “treatments.”
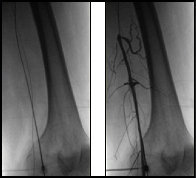
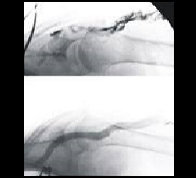
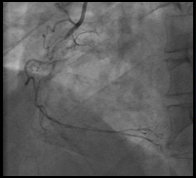
Accessing distal anatomy, navigating tortuous vessels, crossing lesions, and working through blockages with guidewires can be challenging.
These challenges vary based on the vessel type, size, location, and disease morphology. Some procedures benefit from slow clockwise and counterclockwise guidewire control, other cases need high-torque guidewire maneuvering.
Basic torque devices are easy-to-use, but they provide limited in torque, predictability, speed, control, and overall performance. Electromechanical may improve performance for certain procedures, but they are complex, expensive, and often lack tactile feel.
SPINR™ High-Performance Controllers deliver high-torque with predictable clockwise and counterclockwise control.
No motors, electricity, or capital equipment.
Clinicians control the SPINR’s actuation with their hand based on what they see and feel during the procedure.
Squeezing the front handle rotates the guidewire clockwise 5X, releasing the front handle rotates the guidewire counterclockwise 5X. Clinicians control rotational speed and advancement or withdraw throughout the procedure. Physicians may also use their high-torque 0.014”-0.035” guidewire of choice for the situation.
· Predictable clockwise & counterclockwise rotations
· 5X clockwise & counterclockwise rotations per squeeze
· Improved torque
· Variable speed high-performance control
· 0.014” – 0.035” guidewire compatibility
· Peripheral vasculature
PILOT ACCESS & BIOPSY ™
Bone access and percutaneous biopsy may be performed with a variety of devices, systems, and techniques including basic manual devices and electromechanical drill-like systems.
Basic manual devices are cost-effective and easy-to-use, but often lack speed, power, control, and effectiveness. Electromechanical systems may improve speed and power for certain cases, but require costly capital equipment, service contracts, complex set-up, and expensive disposables. Some electromechanical systems reduce physician tactile “feel” for the procedure and may introduce new risks that outweigh their benefit.
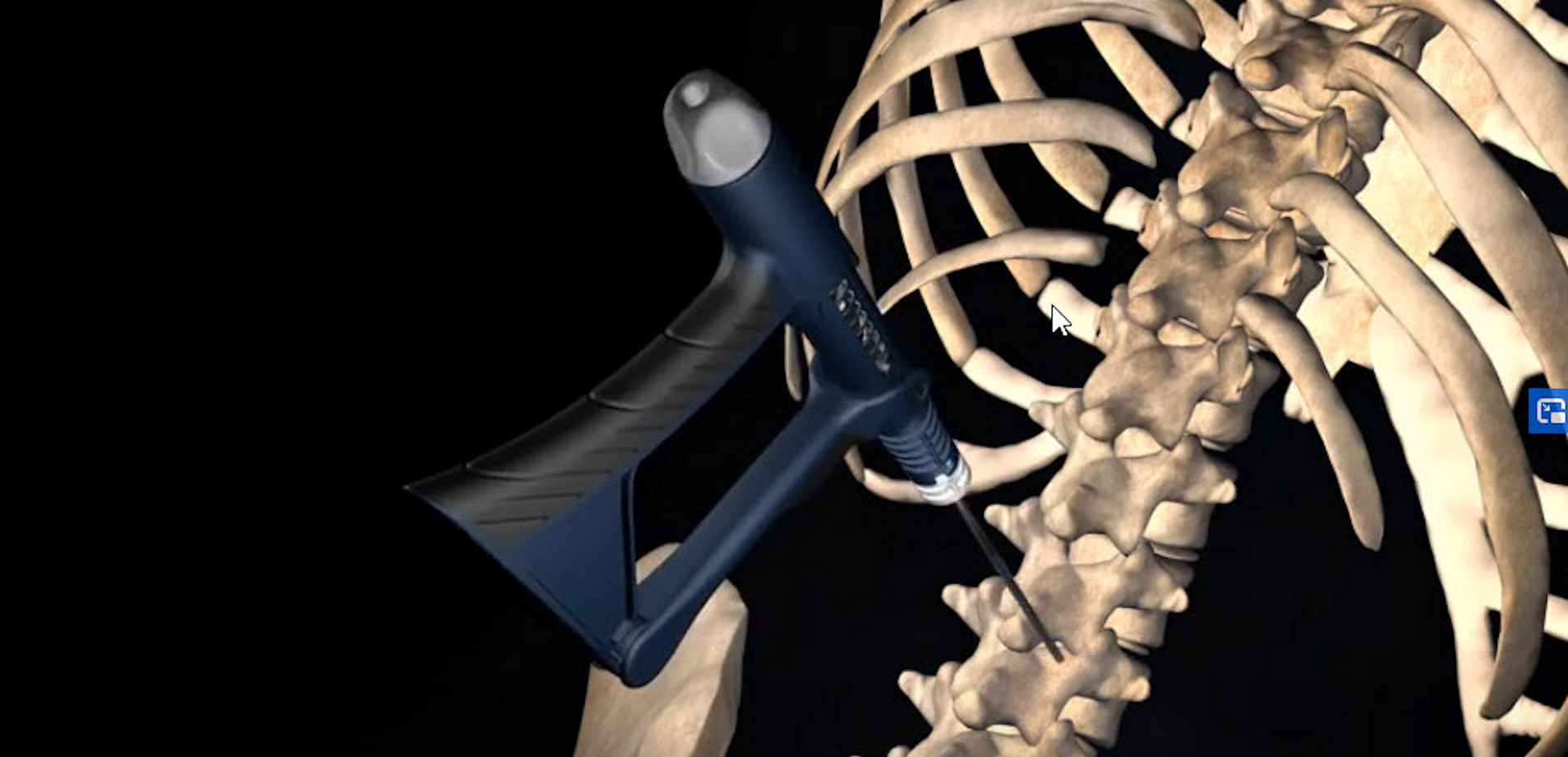
The PILOT is a single-use, non-powered, hand-held, and hand-manipulated tool designed to combine the benefits of basic manual devices and electromechanically powered systems.
Clinicians squeeze and release the handle with their fingers to actuate. Improved control means physicians can perform percutaneous biopsy and/or bone access based on what they see and feel during the procedure.
INDICATIONS FOR USE: The PILOT Access & Biopsy 11ga 10cm device is a non-powered, hand-held, and hand-manipulated manual surgical instrument designed for bone access and percutaneous biopsy. PILOT devices are “tools” not “treatments.”

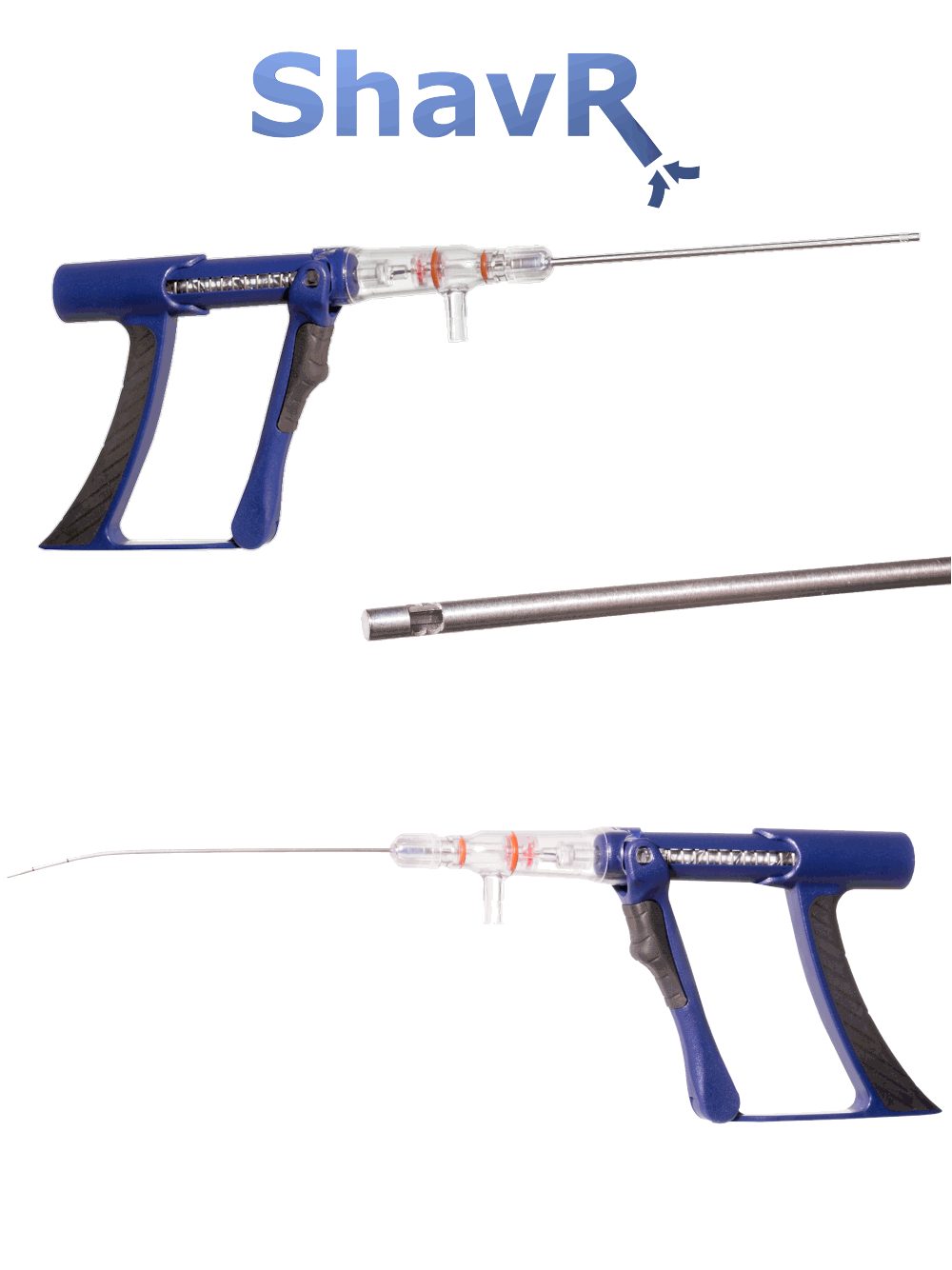
SHAVR
Tissue and/or pathology resection may be performed with a variety of surgical instruments, systems, and techniques including basic manual devices and electromechanical systems.
Basic manual devices are cost-effective and easy-to-use, but often lack speed, power, control, and effectiveness. Electromechanical systems may improve speed and power for certain cases, but require costly capital equipment, service contracts, complex set-up, and expensive disposables. Some electromechanical systems reduce physician tactile “feel” for the procedure and may introduce new risks that outweigh their benefit.
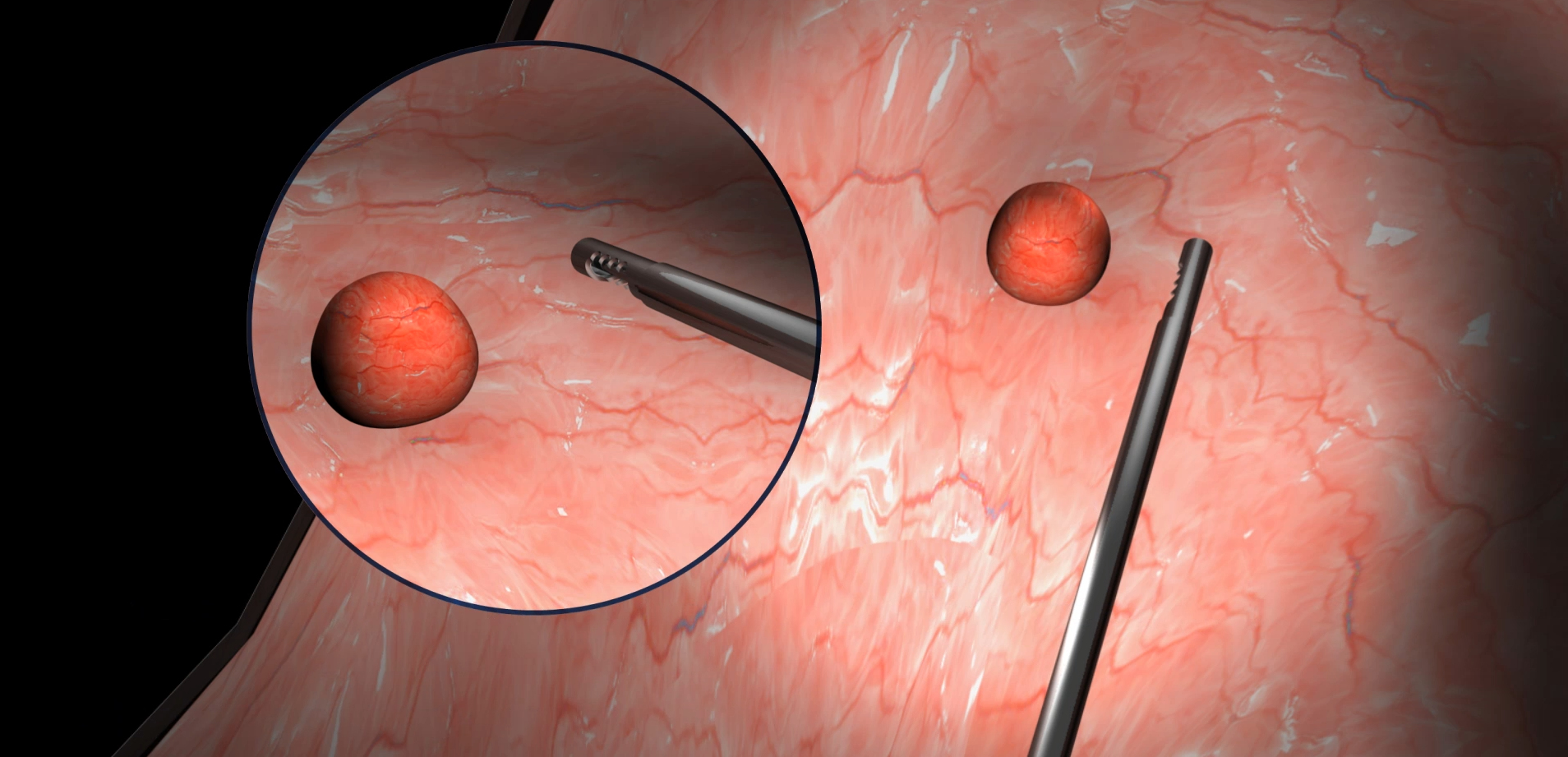
The SHAVR is a single-use, non-powered, hand-held, and hand-manipulated tool designed to combine the benefits of basic manual devices and electromechanically powered systems.
Clinicians squeeze and release the handle with their fingers to actuate cutting speed and control. Aspiration may be provided by wall suction and/or an aspiration pump. Improved control means physicians can perform surgical resection based on what they see and feel during the procedure.
INDICATIONS FOR USE: The Shavr is a non-powered, hand-held, and hand-manipulated manual surgical instrument for tissue resection. Shavr devices are “tools” not “treatments.”
ABOUT DISTAL ACCESS
DISTAL ACCESS
Distal Access designs and develops medical devices to improve patient care including:
Medical Device Controllers
Tissue Resectors, Macerators & Cleaners
Access & Biopsy Devices
Medical devices can help clinicians improve patient care. New technology is often developed with expensive complex electromechanical drive systems that increase speed & power, but often are cost-prohibited and result in less physician tactile feel for the procedure.
Distal Access’s platform is designed to improve performance and maintain physician feel and control for procedure with cost-effective devices. The SPINR driver is a core innovation at Distal Access that allow clinicians to increase torque, speed, power, and/or control with the power of the clinician’s hand.
OUR TEAM
SHAWN FOJTIK
CEO
Park City, Utah USA
LAWRENCE KRONICK
Director of International Sales
Hallandale Beach, Florida USA
TIM NIEMAN
Engineering
Salt Lake City, UT USA
ERIK LIDDIARD
Engineering
Salt Lake City, UT USA
THIAGO HASSEGAWA
Brazil Country Manager
Sao Paulo, Brazil
DIETER HOEVEL
European Manager
Munich, Germany
M.A. SUDHEER
India Country Manager
Bengaluru, India
ALAN YANG
China Manager
SHINYA MIIKE
Japan Manager
Tokyo, Japan
SAM KANAZAWA
Japan Manager
Tokyo Japan
CAROLINA GREGORIO
Latin America Sales Manager &
Global Sales Support
Hallandale Beach, Florida USA
CARMEN AGUILERA
Order Administrator & Trade Shows
Hallandale Beach, Florida USA